Nonpathogenic, Environmental Fungi Induce Activation and Degranulation of Human Eosinophils
Yoshinari Inoue,* Yoshinori Matsuwaki,* Seung-Heon Shin,* Jens U. Ponikau,† and Hirohito Kita2*
Eosinophils and their products are probably important in the pathophysiology of allergic diseases, such as bronchial asthma, and in host immunity to certain organisms. An association between environmental fungal exposure and asthma has been long recognized clinically. Although products of microorganisms (e.g., lipopolysaccharides) directly activate certain inflammatory cells (e.g., macrophages), the mechanism(s) that triggers eosinophil degranulation is unknown. In this study we investigated whether human eosinophils have an innate immune response to certain fungal organisms. We incubated human eosinophils with extracts from seven environmental airborne fungi (Alternaria alternata, Aspergillus versicolor, Bipolaris sorokiniana, Candida albicans, Cladosporium herbarum, Curvularia spicifera, and Penicillium notatum). Alternaria and Penicillium induced calcium-dependent exocytosis (e.g., eosinophil-derived neurotoxin release) in eosinophils from normal individuals. Alternaria also strongly induced other activation events in eosinophils, including increases in intracellular calcium concentration, cell surface expression of CD63 and CD11b, and production of IL-8. Other fungi did not induce eosinophil degranulation, and Alternaria did not induce neutrophil activation, suggesting specificity for fungal species and cell type. The Alternaria-induced eosinophil degranulation was pertussis toxin sensitive and desensitized by preincubating cells with G protein-coupled receptor agonists, platelet-activating factor, or FMLP. The eosinophil-stimulating activity in Alternaria extract was highly heat labile and had an Mr of 60 kDa. Thus, eosinophils, but not neutrophils, possess G protein-dependent cellular activation machinery that directly responds to an Alternaria protein product(s). This innate response by eosinophils to certain environmental fungi may be important in host defense and in the exacerbation of inflammation in asthma and allergic diseases. The Journal of Immunology, 2005, 175: 5439–5447.
*Departments of Immunology and Medicine and †Department of Otorhinolaryngology, Mayo Clinic, Rochester, MN 55905
Received for publication June 2, 2005. Accepted for publication August 15, 2005.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
- This work was supported in part by National Institutes of Health Grant AI49235 and The Mayo Foundation.
- Address correspondence and reprint requests to Dr. Hirohito Kita, Department of Immunology, Mayo Clinic, Rochester, MN 55905. E-mail address: kita. hirohito@mayo.edu
- Abbreviations used in this paper: MBP, eosinophil granule major basic protein; [Ca2]i, intracellular concentration of Ca2; EDN, eosinophil-derived neurotoxin; PA, protease activated; PAB, PBS containing 0.1% NaN3 and 1% BSA; PAF, platelet-activating factor; PAR-2, protease-activated receptor 2; PTX, pertussis toxin.
Eosinophils are implicated in the pathophysiology of allergic diseases, such as bronchial asthma and atopic dermatitis, and in host immunity to helminth infections (1). During such inflammatory reactions, soluble mediators released by immune cells induce eosinophil recruitment from the bloodstream into sites of inflammation, where as yet unknown stimuli trigger the release of eosinophil granule proteins (2). Eosinophil granule major basic protein (MBP)3 and eosinophil peroxidase are toxic to respiratory epithelial cells, pneumocytes, and tracheal epithelium in vitro (3– 6). MBP augments the contraction of tracheal smooth muscle induced by acetylcholine in vitro (7), and instillation of MBP causes airway hyper-responsiveness in primates (8). These observations suggest potential roles for these proteins in the pathophysiology of human diseases related to eosinophils. Indeed, marked extracellular deposition of released eosinophil granule proteins is found in specimens from patients who died of asthma and patients with chronic rhinosinusitis and atopic dermatitis (9 –11). However, the presence of eosinophils per se, as in normal intestinal mucosa (12), does not lead to disease pathology. Thus, a fundamental and important question still remains: what triggers eosinophil activation and proinflammatory mediator release in human disease?
Unlike mast cells and basophils, there is no or only minimal surface expression of FcRI in eosinophils, and eosinophil mediator release is not triggered through the IgE/FceRI interaction (13, 14). In vitro, eosinophil degranulation follows engagement of the IgG and IgA receptors and follows stimulation with soluble in- flammatory mediators such as IL-5, GM-CSF, RANTES, eotaxin, IFN-y, platelet-activating factor (PAF), C5a, and plasma-activated zymosan (15–21). It is not known whether these factors are responsible for eosinophil mediator release in vivo.
Fungi are ubiquitous in the environment, and as saprophytes or commensals, they may coexist without effect in the host with normal cellular immunity (22). Nonetheless, these airborne fungi and their products may contribute to the development and exacerbation of allergic airway diseases. For example, fungal products, e.g., proteins, induce immunologic and inflammatory reactions, resulting in a Th2-like cytokine response and the destruction of mucosal barrier functions (23–25). Clinically, an association between fungal exposure and asthma has been widely recognized (26). Increased spore counts and fungal Ag levels correlate with allergic symptoms (27–29). Moreover, exposure to Alternaria is a risk factor for respiratory arrest in patients with asthma (30). The general consensus at present is that Th2 cell-dependent, Ag-mediated immune responses are probably central mechanisms directing eosinophilic inflammation and disease exacerbations in these conditions. However, direct activation of eosinophils by fungal products may provide another explanation.
In this study we hypothesized that when eosinophils recognize the products of certain common environmental fungi, these cells release their inflammatory mediators. Our results suggest that eosinophils, but not neutrophils, are equipped with innate cellular activation machinery that responds to the products of Alternaria and Penicillium. Exposure of humans to and subsequent activation of eosinophils by these common environmental fungi may provide an important mechanism for exacerbation of eosinophil-related airway disorders, such as asthma.
Materials and Methods
Materials
Culture filtrate extracts from seven different fungi (Alternaria alternata, Aspergillus versicolor, Bipolaris sorokiniana, Candida albicans, Cladosprium herbarum, Curvularia spicifera, and Penicillium notatum) and mycelium extract (cellular extract) of A. alternata were purchased from Greer Laboratories. Culture filtrate extracts are derived from the media in which the fungi were grown; as they grow, they excrete proteins into the media. After removing the medium components, the culture filtrates are concentrated, dialyzed, and lyophilized. Mycelium extract was prepared by extracting the acetone-washed crude mycelium material in a buffer solution; this solution was then lyophilized. RPMI 1640 medium was purchased from Protide Pharmaceuticals. EGTA, thapsigargin, and Nonidet P-40 were obtained from Sigma-Aldrich. Pertussis toxin (PTX) and PMA were obtained from Calbiochem; PAF was purchased from BIOMOL. Stock solutions of thapsigargin (20 mM) were prepared in DMSO; aliquots were stored at 20°C; stock solutions were diluted in HBSS medium with 25 mM HEPES and 0.01% gelatin (Sigma-Aldrich) immediately before use. Anti-CD11b, anti-CD63, and control Ab were purchased from BD
Biosciences. Anti-TLR2 and anti-TLR4 Abs were obtained from eBioscience.
Eosinophil and neutrophil isolation
Human eosinophils were isolated from normal volunteers or patients with histories of asthma, allergic rhinitis, or both by Percoll density gradient centrifugation and MACS using MACS anti-CD16 microbeads as described previously (31). The purity of eosinophils was regularly 98%. Isolated granulocytes (before addition of anti-CD16) were used as neutrophils with purities regularly 92%; neutrophils were further gated electronically during flow cytometric analysis (see below). The Mayo Clinic Rochester institutional review board approved the protocol to obtain blood from volunteers; all provided informed consent.
Eosinophil degranulation assays
To monitor eosinophil function in response to extracts from fungi, IL-5, PAF, or PMA, we measured degranulation of human eosinophils by quantitating released eosinophil-derived neurotoxin (EDN) and MBP (one set of experiments), as described previously (32). In brief, freshly isolated eosinophils were suspended in HBSS with 25 mM HEPES and 0.01% gelatin at 5 105 cells/ml. Eosinophils and stimuli were incubated in 96-well tissue culture plates for 3 h at 37°C and 5% CO2. Cell-free supernatants were stored at 20°C. A specific RIA quantitated eosinophil degranulation by measuring the concentration of EDN in the supernatants (32). MBP release was also measured by two-site immunoradiometric assay (33). Because MBP attaches to plastic and is difficult to detect in the supernatants at neutral pH, after stimulation, MBP was measured by lysing the cell pellet with Nonidet P-40. The percentage of total MBP was calculated as follows: % of total MBP (total MBP in lysate of eosinophils before incubation MBP in lysate after stimulation)/total MBP in lysate of eosinophils before incubation 100%.
To examine the calcium dependency of eosinophil degranulation, cells were preincubated with 1 mM EGTA or 0.5 M thapsigargin for 15 min at 37°C before stimulation with fungal extracts. To investigate the roles of TLR, PTX-sensitive G proteins, and G protein mediation in the eosinophil response to fungi, we preincubated the cells with blocking Abs (10 g/ml) to TLR2 or TLR4 for 30 min, with 100 ng/ml PTX for 2 h at room temperature, or with suboptimal concentrations of PAF (0.3 M) or FMLP (0.1 M) for 15 min before stimulation with fungal products, respectively. To investigate the effects of temperature on Alternaria, the extract and IL-5 solutions were exposed to 4, 37, 56, or 100°C for 30 min and were restored to 37°C before use as stimulants for degranulation. To investigate the degranulation capabilities of the retentates, filtrates, and fractions from the Alternaria characterization experiments (see below), portions of these solutions were incubated with eosinophils for 3 h at 37°C in 5% CO2, and EDN release was quantitated as described above.
Eosinophil morphology
We used transmission electron microscopy to examine eosinophil morphology after culture with Alternaria extract. Isolated eosinophils were incubated with 100 g/ml fungal product in HBSS buffer with gelatin for 3 h at 37°C in 5% CO2. After overnight fixation in 4% formaldehyde and 1% glutaraldehyde in 0.1 M phosphate buffer (pH 7.2), the cell pellet was rinsed, postfixed with 1% phosphate-buffered osmium tetroxide for 60 min, and stained en bloc with 2% aqueous uranyl acetate at 60°C. After dehydration in ethanol, the cells were infiltrated with Spurr resin/ethanol mixtures (1/1 and 3/1) for 60 min each, resuspended in fresh Spurr resin overnight, and embedded in polyethylene capsules. Thin sections were examined with a transmission electron microscope (JEOL 1200).
IL-8 production by eosinophils
Purified eosinophils (1 ml at 1 106 cells/ml) were cultured for 24 h at 37°C in 5% CO2 in RPMI 1640 with 10% bovine calf serum and Alternaria extract. Levels of IL-8 in the cell-free supernatants were measured using an ELISA kit (Quantikine IL-8 Immunoassay Kit; R&D Systems); the threshold sensitivity was 4 pg/ml.
Flow cytometric analyses for CD11b and CD63 expression
Purified eosinophils or granulocytes (1 106cells) were suspended in RPMI 1640 with 25 mM HEPES, 1% BSA, and 0.1% NaN3 and were incubated with Alternaria extract (50 g/ml) or PAF (1.0 M) as a positive control for 1 h at 37°C. After washing with PBS containing 0.1% NaN3 and 1% BSA (PAB buffer), cells were incubated for 30 min at 4°C with anti-CD11b, anti-CD63, or control Ab, followed by incubation with PE-conjugated goat anti-mouse IgG. After washing with PAB buffer, cells were fixed with 1% paraformaldehyde in PAB buffer (pH 7.4). The fluorescence intensity of individual cells was measured with a FACScan (BD Biosciences). In the granulocyte preparation, neutrophils were identified by their weaker green autofluorescence and side scatter, as previously described (13)
Measurement of intracellular Ca2
Real-time changes in intracellular Ca2 ([Ca2]i) were measured in a flow cytometer (34) using the calcium indicator indo-1 (35). To load the eosinophils, a 1-ml suspension (1 to 2 106 cells/ml) was incubated with 3 mM indo-1-AM (Molecular Probes) in phenol red-free RPMI 1640 with 10% calf serum and 10 mM HEPES for 30 min at 37°C. After washing, cells were suspended in RPMI 1640 with 0.1% human serum albumin and 10 mM HEPES. To measure [Ca2]i, cells were stimulated, and fluorescence was analyzed by a FACS analyzer with an ion-argon laser (BD Biosciences). [Ca2]i was monitored on the basis of the ratio of the fluorescence of the calcium-bound indo-1 emission (401 nm) and the free indo-1 emission (475 nm).
Size exclusion chromatography of Alternaria culture extract
To probe the components in Alternaria extract that are involved in eosinophil activation, we used size exclusion Chromatography with a Superdex 200–10/30 column (Amersham Biosciences). A vial of A. alternata culture filtrate Ag (38 mg/vial; 15.4% protein) was mixed with 1 ml of 0.05 M PO4 buffer, pH 6.8, sonicated in a water bath for 2 min at 22°C, and applied to a YM100 Centricon membrane system (100-kDa cutoff; Millipore). After centrifugation at 3000 g for 1 h at 4°C, the resulting filtrate was applied to a YM10 Centricon membrane system (10-kDa cutoff). After centrifugation at 3000 g for 6 h at 4°C, the retentate was applied to the column and eluted with 0.05 M PO4 at a flow rate of 0.5 ml/min; 0.5-ml fractions were collected. The UV absorbance of fractions was measured by SpectraMAX plate reader (Molecular Probes), and their abilities to induce eosinophil degranulation were determined by EDN release (see above).
Statistics
Data from three or more experiments using eosinophil preparations from different donors were summarized as the mean SEM. Statistical analyses (two-tailed) used Student’s t, Mann-Whitney U, or Wilcoxon test.
Results
Alternaria induces exocytotic degranulation of human eosinophils We investigated whether the culture extracts of seven common environmental fungi (A. alternata, A. versicolor, B. sorokiniana, C. albicans, C. herbarum, C. spicifera, and P. notatum) induce
degranulation of human eosinophils in vitro. Instead of live fungi, we used commercial, lyophilized products; this minimized experimental variability from day-to-day differences in the growth stages of each fungus. Both Alternaria and Penicillium induced concentration-dependent EDN release, as a marker of eosinophil degranulation (Fig. 1). Other fungi, including Aspergillus, Bipolaris, Candida, Cladosporium, and Culvularia up to 200 g/ml, induced no or minimal EDN release. The Alternaria- and Penicillium- induced degranulation increased with time (results not shown). After 3-h incubation, Alternaria (50 g/ml) and Penicillium (200 g/ml) induced maximal EDN release (263 60 and 237 60 ng EDN/2.5 105 cells, respectively) or 30% of total cellular EDN. As potent eosinophil secretagogues, PAF (1 M) and PMA (1 ng/ml) induced 547 32 and 402 35 ng EDN/ 2.5 105 cells of EDN release, respectively. Alternaria also stimulated the release of another eosinophil granule protein, MBP; eosinophils incubated for 3 h with Alternaria (100 g/ml) released 52 5% of their total MBP (mean range; n 2).
 |
|
FIGURE 1. Effects of fungi on eosinophil degranulation. Eosinophils were incubated in duplicate with different concentrations of culture extracts from fungi (A. alternata, A. versicolor, B. sorokiniana, C. albicans, C. herbarum, C. spicifera, or P. notatum) for 3 h at 37°C. EDN concentrations in the cell-free supernatants were measured by RIA, as described in Materials and Methods. Results show the mean SEM from six different eosinophil preparations. , Significant differences compared with medium alone (p 0.05). |
By electron microscopy, eosinophils incubated at 37°C for 3 h with Alternaria extract (100 g/ml) showed granule fusion and electron-lucent granule cores and matrices (Fig. 2), consistent with the extracellular release of both core (MBP) and matrix (EDN) granule proteins (see above). The plasma membranes remained mostly intact, suggesting that Alternaria induced compound exocytosis (36) of eosinophils. In contrast, most eosinophils incubated in medium maintained their cytoplasmic granules with characteristic core and matrix structures and intact plasma membranes.
|
|
|
|
|
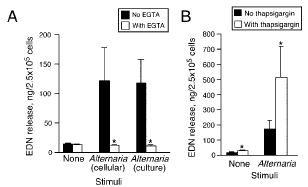
FIGURE 3. Effects of calcium on Alternaria-induced eosinophil degranulation. Eosinophils were preincubated with 1 mM EGTA (A) or 0.5 M thapsigargin (B) for 15 min at 37°C and stimulated with medium, 100 g/ml Alternaria cellular, and culture extracts for 3 h at 37°C. Results show the mean SEM from five (A) and six (B) different eosinophil preparations. , Significant differences compared with no EGTA (A) or no thapsigargin (B; p 0.05). |
Although the molecular mechanisms for eosinophil exocytosis are incompletely understood, increased [Ca2]i is a key triggering step in the coupling of stimulus to secretion (36). Therefore, we studied the roles of [Ca2]i and extracellular Ca2. Both culture and cellular extracts of Alternaria induced eosinophil EDN release, but eosinophils that were preincubated with 1 mM EGTA did not degranulate (Fig. 3A), suggesting that Alternaria-induced eosinophil degranulation is highly dependent on extracellular Ca2. We next investigated the priming effects of a well-defined agonist that increases [Ca2]i. Thapsigargin inhibits the endoplasmic reticulum Ca2-ATPase and allows influx to the cytoplasm, thus elevating [Ca2]i from intracellular stores (37). Eosinophils pretreated with suboptimal concentrations of thapsigargin showed synergistic and dramatic increases in Alternaria-induced degranulation (Fig. 3B). Thus, extracellular Ca2 and [Ca2]i probably play key roles in Alternaria-induced eosinophil degranulation.
|
|
|
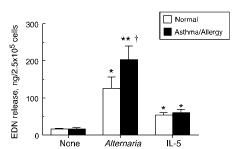
FIGURE 4. Comparison of eosinophil degranulation with cells from healthy donors and from patients with asthma or allergy. Purified eosinophils from 10 normal volunteers and eight volunteers with asthma or allergy or both were incubated with 100 g/ml Alternaria or 10 ng/ml IL-5 for 3 h. EDN concentrations in the cell-free supernatants were measured by RIA, as described in Materials and Methods. Results show the mean SEM. , p 0.05; , p 0.01 (significant difference compared with medium). †, p 0.05 (significant difference, normal compared with asthma/allergy). |
Alternaria induces eosinophil exocytosis, but is this response limited to Alternaria-sensitized individuals? Acid stripping of eosinophils with lactic acid to remove cell-bound IgE and IgG (13) did not affect Alternaria-induced eosinophil degranulation (data not shown). Furthermore, Alternaria induced degranulation of eosinophils isolated from normal individuals ( p 0.01; n 10; Fig. 4), suggesting that this response to Alternaria is not limited to sensitive patients. However, eosinophils from patients with clinical allergy or asthma released 70% more EDN compared with normal individuals ( p 0.05; n 8 and n 10, respectively). No difference was observed in IL-5-induced EDN release between these groups.
Alternaria induces IL-8 production in and CD11b up-regulation on eosinophils
Other effector functions of eosinophils include the production and release of various proinflammatory cytokines and chemokines, including IL-8 (38). Eosinophils incubated with Alternaria for 24 h produced IL-8 in their supernatants (Fig. 5A), but IL-5-stimulated cells did not. Lysates from freshly isolated eosinophils (prepared using 0.5% Nonidet P-40) showed no detectable IL-8 (data not shown), suggesting de novo synthesis of IL-8 when stimulated with Alternaria.
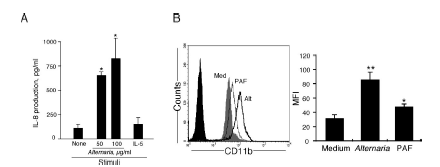 |
|
FIGURE 5. Alternaria induces IL-8 from and CD11b on eosinophils. A, Eosinophils were incubated with 50 or 100 g/ml Alternaria extract or 10 ng/ml IL-5 for 24 h at 37°C. The levels of IL-8 in the supernatants were measured by ELISA. Results show the mean SEM from three different eosinophil preparations. , p 0.05 (significant difference compared with medium). B, Eosinophils were incubated with 50 g/ml Alternaria extract, 1.0 M PAF, or medium for 1 h. A representative histogram illustrates the differences in surface expression for cells stimulated with Alternaria (thick line) , PAF (thin line), medium (light gray area), and isotype control Ab (dark gray area). Bar graphs show the results of three ndependent experiments. , p 0.05; , p 0.01 (significant differences compared with medium). |
Stimulation of eosinophils with their agonists, such as PAF and FMLP, up-regulates surface expression of a 2 integrin, CD11b, and this increased CD11b expression is an activation marker for eosinophils (39). We next investigated the effects of Alternaria on eosinophil CD11b expression. Cells stimulated with 50 g/ml Alternaria or 1 M PAF, as a positive control, showed increased expression of eosinophil CD11b compared with medium (Fig. 5B, left panel). A summary of three experiments (Fig. 5B, right panel) shows that Alternaria highly increased the expression of CD11b, even more than PAF. Alternaria probably triggers various effector functions of eosinophils, including exocytosis, chemokine production, and integrin expression.
Alternaria stimulates CD63 expression by eosinophils, but not by neutrophils
Both eosinophils and neutrophils share a number of cellular receptors for microbial products (e.g., zymosan and FMLP), and some receptors are preferentially expressed by neutrophils (e.g., TLR2, TLR4, and TLR5) (40). Therefore, we compared the eosinophil and neutrophil cellular responses to Alternaria. CD63 is a well-established component of the late endosomal and lysosomal membranes (41) and is used as a surface marker for exocytosis in both eosinophils and neutrophils (19, 42). Both 50 g/ml Alternaria and 1.0 M PAF increased eosinophil surface expression of CD63 (Fig. 6A). In contrast, PAF, but not Alternaria, increased the expression of CD63 in neutrophils (Fig. 6B). Thus, the activation response to Alternaria occurs in eosinophils, but it is unlikely in neutrophils.
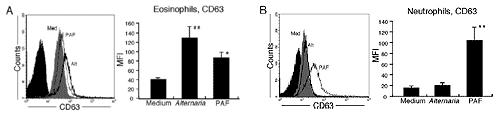 |
|
FIGURE 6. Alternaria-induces CD63 expression on eosinophils and not neutrophils. Purified eosinophils (A) or purified neutrophils (B) were incubated with 50 g/ml Alternaria extract and 1.0 M PAF or medium for 1 h. Representative histograms are described in Fig. 5. Bar graphs show the results of three independent experiments. , p 0.05; , p 0.01 (significant differences compared with medium). |
Potential role of heterotrimeric G protein(s) in the eosinophil response to Alternaria
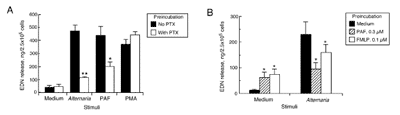
We next investigated how eosinophils recognize Alternaria products. Initially, we examined the effects of blocking Abs, including anti-TLR2 and anti-TLR4. These Abs inhibited Alternaria-induced degranulation by 10% (data not shown), suggesting that TLR involvement is unlikely. Ca2 is strongly implicated in eosinophil exocytosis (20) (see Fig. 3); thus, exposure to Alternaria might induce increased [Ca2]i. After loading cells with the calcium-sensitive fluorescent dye, indo-1, we monitored the [Ca2]i changes in stimulated eosinophils by flow cytometry. Eosinophils incubated with 10 ng/ml IL-5 or medium showed no change in [Ca2]i (Fig. 7). In contrast, eosinophils stimulated with 100 g/ml Alternaria showed rapid increases in [Ca2]i within 200 s; the increased [Ca2]i persisted for up to 500 s, suggesting the involvement of a calcium-mobilizing receptor(s), such as a G protein-coupled receptor(s). Next, we preincubated eosinophils with PTX for 2 h and stimulated cells with Alternaria for 3 h. Because the PAFR is coupled to PTX-sensitive G protein in human eosinophils (43), 1.0 M PAF was used as a positive control. PTX treatment significantly inhibited both PAF- and Alternaria-induced EDN release (60% ( p 0.05; n 5) and 80% ( p 0.01; n 5), respectively; Fig. 8A). PMA acts independently of G proteins, and 1 ng/ml PMA was used as a second positive control; PTX had no effect on PMA-induced eosinophil degranulation. We next investigated whether the eosinophil’s response to Alternaria is consistent with G protein mediation by manifesting the phenomenon of heterologous desensitization (44, 45). Eosinophils were preincubated with suboptimal concentrations of PAF or FMLP for 15 min and then stimulated with medium or 100 g/ml Alternaria for 3 h. Cells incubated with PAF or FMLP without Alternaria showed small, but significant, EDN release (70 ng EDN/2.5 105 cells; p 0.05; n 4; Fig. 8B). Without PAF or FMLP pretreatment, Alternaria induced the release of 225 ng EDN/2.5 105 cells. Pretreatment with PAF decreased this Alternaria-induced EDN release to 90 ng EDN/2.5 105 cells ( p 0.05; n 4), a level comparable to that after PAF pretreatment without Alternaria. Similarly, pretreatment with FMLP partially decreased the Alternaria-induced EDN release ( p 0.05). Thus, a G protein-coupled receptor(s), probably a PTX-sensitive Gi-coupled receptor(s), is likely to be involved in the eosinophil’s response to Alternaria.
Partial characterization of Alternaria extract
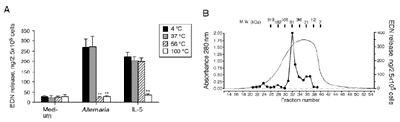
We used three strategies to begin characterizing the Alternaria products involved in eosinophil degranulation. First, the Alternaria extract was subjected to membrane filtration. After filtration with a YM100 Centricon membrane, the filtrate stimulated eosinophil degranulation, but the retentate did not (results not shown). After filtration with a YM10 Centricon membrane, the retentate stimulated eosinophil degranulation, but the filtrate did not. Thus, the eosinophil stimulatory activity in the Alternaria extract is probably between 10 and 100 kDa. Second, Alternaria extracts, which had been treated at 56 or 100°C for 30 min, did not induce EDN release (Fig. 9A), but extracts treated at 4 or 37°C for 30 min did induce EDN release, suggesting that it is a heat-labile protein(s) or glycoprotein(s). The activity of a cytokine, IL-5, to induce EDN release was abolished by treatment at 100°C, but not by treatment at 56°C or lower temperatures. Third, we used size exclusion chromatography (Fig. 9B) and tested the column fractions for their abilities to induce eosinophil degranulation. Although the absorbance profile showed a broad peak from fractions 32–37, the most potent eosinophil degranulation activity appeared in fraction 32 with an Mr of 60 kDa.
Discussion
Although recent studies by several investigators have elucidated innate immune responses of various inflammatory cells to microorganisms, the innate immune responses of human eosinophils remain unknown. Unlike macrophages or neutrophils, the reported TLR expression on eosinophils is limited, except for TLR7 (40). Our report is the first to show that products of fungi (i.e., Alternaria and Penicillium) induce in vitro activation and degranulation of human eosinophils. This Alternaria-induced exocytosis of eosinophils is highly dependent on extracellular Ca2 and [Ca2]i and is mediated by PTX-sensitive G proteins. In addition, Alternaria culture extract induced synthesis of IL-8 and increased the expression of CD11b and CD63, suggesting that a series of activation events, including exocytosis, integrin expression, and cytokine production, follows the exposure of eosinophils to Alternaria products. Together, human eosinophils probably react with certain fungi, such as Alternaria and Penicillium, as part of their role in innate immunity.
![Changes in [Ca2]i in eosinophils stimulated with medium, Alternaria culture extract, or IL-5](https://eii-lucid.s3.amazonaws.com/clinicoffps/nonpathogenic%20fungi8.gif) |
|
FIGURE 7. Changes in [Ca2]i in eosinophils stimulated with medium, Alternaria culture extract, or IL-5. Eosinophils were pretreated with the calcium-sensitive fluorescent dye indo-1-AM, loaded onto the FACS analyzer, and stimulated after 20 s with medium, 100 g/ml Alternaria culture extract, or 10 ng/ml IL-5. [Ca2]i is shown as the ratio of the calciumbound |
The potential implications of our study in understanding the mechanisms of asthma and other allergic diseases may be substantial. Previous studies suggest that T cell-mediated immune responses to exogenous Ags, such as mite and cockroach, and coordinated actions by cytokines, chemokines, and adhesion molecules recruit eosinophils to the airways (2); however, the triggers of proinflammatory mediator release by eosinophils in the airways are unknown. Importantly, unlike mast cells and basophils, the expression of IgE receptors on eosinophils is extremely limited (13, 14). Our observations suggest that products of certain environmental fungi, such as Alternaria and Penicillium, may directly induce exocytotic release of granule proteins from eosinophils in the absence of other immune cells or Igs. An association between fungal exposure and asthma has long been recognized clinically (26, 27). Furthermore, an accumulating body of evidence suggests that sensitivity to fungi, particularly Alternaria, is associated with asthma (26). Alternaria is ubiquitous both outdoors and indoors (46) and is known for the high rate of its spore germination and Ag release (47). Sensitization to Alternaria has been associated with asthma in various countries and in regions of the United States (48, 49). Moreover, exposure to Alternaria is a risk factor for respiratory arrest in patients with asthma (30). Similar reports have indicated that sensitivity to fungal proteins is a significant risk factor for lifethreatening asthma (50). Therefore, the orchestration of both the acquired immune response (e.g., Th2 cytokine response) to
 |
|
FIGURE 8. Alternaria-induced eosinophil activation involves a PTXsensitive, G-protein-coupled receptor. A, After a 2-h preincubation with 100 ng/ml PTX or medium, eosinophils were stimulated for 3 h with 100 g/ml Alternaria, 1.0 M PAF, or 1.0 ng/ml PMA. B, After a 15-min preincubation with suboptimal concentrations of PAF or FMLP or with medium, eosinophils were stimulated with 100 g/ml Alternaria for 3 h. Results show the mean SEM from five (A) and four (medium and PAF) or three (FMLP; B) different eosinophil preparations. , (p 0.05; , p 0.01 (significant differences compared with no PTX (A) or medium only (B) preincubation). |
certain fungi (e.g., Alternaria), which mobilizes eosinophils, and the innate direct response by eosinophils to the same fungi, which induces mediator release, may have important implications in the pathophysiology and exacerbation of asthma and other eosinophil-related airway diseases.
We also investigated which component(s) in Alternaria extracts stimulates eosinophils. Because fungal extracts contain large quantities of proteases (51), they could be potential candidates. Although information on fungal proteases is limited, they potently induce epithelial cell desquamation and the production of proin-flammatory cytokines (51). Recently, we demonstrated that human eosinophils express functional protease-activated receptor 2 (PAR- 2), and that serine proteases, such as trypsin, activate effector functions of human eosinophils through this receptor (52). PARs are coupled to a heterotrimeric G protein(s), and the increase in [Ca2]i is an activation hallmark of these receptors (53). In the present study the stimulatory activity of Alternaria extract was present in an 60-kDa fraction and was very heat labile, suggesting that it is probably a protein(s). The rapid increase in [Ca2]i in eosinophils stimulated with Alternaria (Fig. 7), the PTX sensitivity (Fig. 8A), and the heterogeneous desensitization by PAF or FMLP (Fig. 8B) are all consistent with the involvement of a G protein coupled receptor(s). Furthermore, in preliminary studies we found that Alternaria-induced increases in [Ca2]i and EDN release were inhibited 60% by a PAR-2 peptide antagonist, LSIGKV (54) (data not shown). In contrast, no trypsin-like activity was detectable in our Alternaria extract, and an Aspergillus extract did not stimulate eosinophils, but it did contain trypsin-like activity (data not shown). Alternatively, because -D-glucans have been reported to stimulate immune function and proinflammatory activity, perhaps -D-glucan, a primary component of fungal cell walls or secreted products of various fungi, might trigger eosinophil degranulation (55). However, preliminary results showed that various concentrations of -D-glucan did not induce eosinophil degranulation or superoxide production in vitro (data not shown). Finally, certain microorganisms, such as HIV and fungi, might directly interact with or produce a molecule(s) that binds to a cell’s chemokine receptors (e.g., CCR5), which are coupled to certain G proteins (56, 57). Additional studies are needed to identify the stimulatory component(s) in Alternaria extract and its receptor(s) on eosinophils.
 |
|
FIGURE 9. Partial characterization of Alternaria extract. A, Before incubation with eosinophils, aliquots of 100 g/ml Alternaria and 10 ng/ml IL-5 were heated at 37, 56, or 100°C for 30 min or were treated at 4°C for 30 min. Eosinophils were incubated in duplicate with these treated stimuli for 3 h at 37°C. Results show the mean SEM from five different eosinophil preparations. , p 0.01 (significant differences compared with no heat treatment of extract). B, Size exclusion chromatography used a Superdex 200–10/30 column and produced a broad absorbance peak (smooth line) of the Alternaria culture extract. The dots connected by lines show the levels of EDN release when portions of fractions 21–39 were incubated with eosinophils. The Mr calibration of the column is shown above the elution profile. |
The majority of previous studies of antifungal immune responses used the following models: animal infection in in vivo systems (e.g., C. albicans and A. fumigatus) or entire fungal hyphae or conidia (e.g., C. albicans and A. fumigatus), a yeast model (e.g., zymosan), or isolated fungal macromolecules (e.g., -glucan and mannan) in in vitro systems (58). These studies pointed to critical roles for TLRs, in particular TLR2 and TLR4, and to other pattern recognition receptors that recognize fungal pathogens and their cell wall components by immune cells, such as macrophages and neutrophils. Our unique approach used the secreted products of fungi, namely culture extracts, rather than fungal organisms. We found that certain environmental fungi, such as Alternaria and Penicillium, but not Candida or Aspergillus, secrete products that stimulate eosinophils through a G protein-dependent mechanism, leading to cellular activation and effector functions; neutrophils did not show a similar response. These findings suggest a novel innate immunological pathway, other than TLRs, that eosinophils use to recognize certain microorganisms and their products. Questions FIGURE 9. Partial characterization of Alternaria extract. A, Before incubation with eosinophils, aliquots of 100 g/ml Alternaria and 10 ng/ml IL-5 were heated at 37, 56, or 100°C for 30 min or were treated at 4°C for 30 min. Eosinophils were incubated in duplicate with these treated stimuli for 3 h at 37°C. Results show the mean SEM from five different eosinophil preparations. , p 0.01 (significant differences compared with no heat treatment of extract). B, Size exclusion chromatography used a Superdex 200–10/30 column and produced a broad absorbance peak (smooth line) of the Alternaria culture extract. The dots connected by lines show the levels of EDN release when portions of fractions 21–39 were incubated with eosinophils. The Mr calibration of the column is shown above the elution
profile remain regarding the specific microbial molecules and cellular receptors involved in this interaction. Additional questions include describing the conditions for fungi to release such bioactive products and whether innate immune cells other than eosinophils (e.g., mast cells) can recognize these products. The physiologic importance of this pathway in human immunity and in disease processes also needs to be elucidated. A better understanding of the interactions between eosinophils and fungi could provide a basis for new therapeutic strategies to prevent the development and exacerbation of asthma and other chronic airway diseases.
Acknowledgments
We thank Debra Ward and LuRaye Eischens for secretarial assistance, Cheryl Adolphson for editorial assistance, and James Checkel and Melinda Miller for size exclusion chromatography.
Disclosures
The authors have no financial conflict of interest.
References
1. Gleich, G. J., and C. R. Adolphson. 1986. The eosinophilic leukocyte: structure and function. Adv. Immunol. 39: 177–253.
2. Kita, H., C. R. Adolphson, and G. J. Gleich. 2003. Biology of eosinophils. In Middleton’s Allergy: Principles and Practice, Vol. 1, 6th Ed. N. F. Adkinson, Jr., B. S. Bochner, J. W. Yunginger, S. T. Holgate, W. W. Busse, and F. E. R. Simons, eds. Mosby, St. Louis, pp. 305–332.
3. Gleich, G. J., E. Frigas, D. A. Loegering, D. L. Wassom, and D. Steinmuller. 1979. Cytotoxic properties of eosinophil major basic protein. J. Immunol. 123: 2925–2927.
4. Ayars, G. H., L. C. Altman, G. J. Gleich, D. A. Loegering, and C. B. Baker. 1985. Eosinophil- and eosinophil granule-mediated pneumocyte injury. J. Allergy Clin. Immunol. 76: 595–604.
5. Gleich, G. J. 1990. The eosinophil and bronchial asthma: Current understanding. J. Allergy Clin. Immunol. 85: 422–436.
6. Motojima, S., E. Frigas, D. A. Loegering, and G. J. Gleich. 1989. Toxicity of eosinophil cationic proteins for guinea pig tracheal epithelium in vitro. Am. Rev. Respir. Dis. 139: 801–805.
7. Brofman, J. D., S. R. White, J. S. Blake, N. M. Munoz, G. J. Gleich, and A. R. Leff. 1989. Epithelial augmentation of trachealis contraction caused by major basic protein of eosinophils. J. Appl. Physiol. 66: 1867–1873.
8. Gundel, R. H., L. G. Letts, and G.J. Gleich. 1991. Human eosinophil major basic protein induces airway constriction and airway hyperresponsiveness in primates. J. Clin. Invest. 87: 1470–1473.
9. Filley, W. V., K. E. Holley, G. M. Kephart, and G. J. Gleich. 1982. Identification by immunofluorescence of eosinophil granule major basic protein in lung tissues of patients with bronchial asthma. Lancet 2: 11–16.
10. Ponikau, J. U., D. A. Sherris, G. M. Kephart, E. B. Kern, D. J. Congdon, C. R. Adolphson, M. J. Springett, G. J. Gleich, and H. Kita. 2005. Striking deposition of toxic eosinophil major basic protein in mucus: Implications for chronic rhinosinusitis. J. Allergy Clin. Immunol. 116: 362–369.
11. Leiferman, K. M., S. J. Ackerman, H. A. Sampson, H. S. Haugen, P. Y. Venencie, and G. J. Gleich. 1985. Dermal deposition of eosinophil-granule major basic protein in atopic dermatitis: comparison with onchocerciasis. N. Engl. J. Med.
313: 282–285.
12. Matthews, A. N., D. S. Friend, N. Zimmermann, M. N. Sarafi, A. D. Luster, E. Pearlman, S. E. Wert, and M. E. Rothenberg. 1998. Eotaxin is required for the baseline level of tissue eosinophils. Proc. Natl. Acad. Sci. USA 95:
6273– 6278.
13. Kita, H., M. Kaneko, K. R. Bartemes, D. A. Weiler, A. W. Schimming, C. E. Reed, and G. J. Gleich. 1999. Does IgE bind to and activate eosinophils from patients with allergy? J. Immunol. 162: 6901–6911.
14. Seminario, M. C., S. S. Saini, D. W. MacGlashan, Jr., and B. S. Bochner. 1999. Intracellular expression and release of FcRIby human eosinophils. J. Immunol. 162: 6893–6900.
15. Abu-Ghazaleh, R. I., T. Fujisawa, J. Mestecky, R. A. Kyle, and G. J. Gleich. 1989. IgA-induced eosinophil degranulation. J. Immunol. 142: 2393–2400.
16. Khalife, J., M. Capron, J. Y. Cesbron, P. C. Tai, H. Taelman, L. Prin, and A. Capron. 1986. Role of specific IgE antibodies in peroxidase (EPO) release from human eosinophils. J. Immunol. 137: 1659–1664.
17. Kroegel, C., T. Yukawa, G. Dent, P. Venge, K. F. Chung, and P. J. Barnes. 1989. Stimulation of degranulation from human eosinophils by platelet-activating factor. J. Immunol. 142: 3518–3526.
18. Elsner, J., R. Hochstetter, D. Kimmig, and A. Kapp. 1996. Human eotaxin represents a potent activator of the respiratory burst of human eosinophils. Eur. J. Immunol. 26: 1919–1925.
19. Mahmudi-Azer, S., G. P. Downey, and R. Moqbel. 2002. Translocation of the tetraspanin CD63 in association with human eosinophil mediator release. Blood 99: 4039–4047.
20. Kernen, P., M. P. Wymann, V. von Tscharner, D. A. Deranleau, P. C. Tai, C. J. Spry, C. A. Dahinden, and M. Baggiolini. 1991. Shape changes, exocytosis, and cytosolic free calcium changes in stimulated human eosinophils. J. Clin.
Invest. 87: 2012–2017.
21. Bach, M. K., and J. R. Brashler. 1992. FMLP is a potent activator of guinea-pig eosinophils but its activity is dependent on the prior overnight in vitro culture of the cells (facilitation). Immunology 75: 680–687.
22. van Burik, J. A., and P. T. Magee. 2001. Aspects of fungal pathogenesis in humans. Annu. Rev. Microbiol. 55: 743–772.
23. H. F. Kaufman, J. F. Tomee, T. S. van der Werf, J. G. de Monchy, and G. K. Koeter. 1995. Review of fungus-induced asthmatic reaction. Am. J. Respir. Crit. Care Med. 151: 2109–2115.
24. Tomee, J. F., and H. F. Kaufman. 2000. Putative virulence factors of Aspergillus fumigatus. Clin. Exp. Allergy 30: 476–484.
25. Kherdmand, F., A. Kiss, J. Xu, S. H. Lee, P. E. Kolattukudy, and D. B. Corry. 2002. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J. Immunol. 169: 5904–5911.
26. Bush, R. K., and J. J. Prochnau. 2004. Case study: Alternaria-induced asthma. J. Allergy Clin. Immunol. 113: 227–234.
27. Dill, I., and B. Niggemann. 1996. Domestic fungal viable propagules and sensitization in children with IgE mediated allergic diseases. Pediatr. Allergy Immunol. 7: 151–155.
28. Li, C. S., and L. Y. Hsu. 1997. Airborne fungus allergen in association with residential characteristics in atopic and control children in a subtropical region. Arch. Environ. Health 52: 72–79.
29. Aas, K., J. Leegard, L. Aukrust, and O. Grimmer. 1980. Immediate type hypersensitivity to common moulds: a comparison of different diagnostic materials. Allergy 35: 443–451.
30. O’Hollaren, M. T., J. W. Yunginger, K. P. Offord, M. J. Somers, E. J. O’Connell, D. J. Ballard, and M. I. Sachs. 1991. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N. Engl.
J. Med. 324: 359–363.
31. Hansel, T. T., I. J. De Vries, T. Iff, S. Rihs, M. Wandzilak, S. Betz, K. Blaser, and C. Walker. 1991. An improved immunomagnetic procedure for the isolation of highly purified human blood eosinophils. J. Immunol. Methods 145: 105–110.
32. Horie, S., and H. Kita. 1994. CD11b/CD18 (Mac-1) is required for degranulation of human eosinophils induced by human recombinant granulocyte-macrophage colony-stimulating factor and platelet-activating factor. J. Immunol. 152: 5457–5467.
33. Wagner, J. M., K. Bartemes, K. K. Vernof, S. Dunnette, K. P. Offord, J. L. Checkel, and G. J. Gleich. 1993. Analysis of pregnancy-associated major basic protein levels throughout gestation. Placenta 14: 671–681.
34. Rabinovitch, P. S., C. H. June, A. Grossmann, and J. A. Ledbetter,. 1986. Heterogeneity among T cells in intracellular free calcium responses after mitogen stimulation with PHA or anti-CD3: simultaneous use of indo-1 and immunofluorescence with flow cytometry. J. Immunol. 137: 952–961.
35. Grynkiewicz, G., M. Poenie, and R. Y. Tsien. 1985. A new generation of Ca2 indicators with greatly improved fluorescence properties. J. Biol. Chem. 260: 3440–3450.
36. Logan, M. R., S. O. Odemuyiwa, and R. Moqbel. 2003. Understanding exocytosis in immune and inflammatory cells: the molecular basis of mediator secretion. J. Allergy Clin. Immunol. 111: 923–932.
37. Thastrup, O., P. J. Cullen, B. K. Drobak, M. R. Hanley, and A. P. Dawson. 1990. Thapsigargin, a tumor promoter, discharges intracellular Ca2 stores by specific inhibition of the endoplasmic reticulum Ca2-ATPase. Proc. Natl. Acad. Sci.
USA 87: 2466–2470.
38. Braun, R. K., M. Franchini, F. Erard, S. Rihs, I. J. De Vries, K. Blaser, T. T. Hansel, and C. Walker. 1993. Human peripheral blood eosinophils produce and release interleukin-8 on stimulation with calcium ionophore. Eur. J. Immunol.
23: 956–960.
39. Kroegel, C., M. C. Liu, W. C. Hubbard, L. M. Lichtenstein, and B. S. Bochner. 1994. Blood and bronchoalveolar eosinophils in allergic subjects after segmental antigen challenge: surface phenotype, density heterogeneity, and prostanoid production. J. Allergy Clin. Immunol. 93: 725–734.
40. Nagase, H., S. Okugawa, Y. Ota, M. Yamaguchi, H. Tomizawa, K. Matsushima, K. Ohta, K. Yamamoto, and K. Hirai. 2003. Expression and function of Toll-like receptors in eosinophils: activation by Toll-like receptor 7 ligand. J. Immunol.
171: 3977–3982.
41. Kobayashi, T., M. H. Beuchat, M. Lindsay, S. Frias, R. D. Palmiter, H. Sakuraba, R. G. Parton, and J. Gruenberg. 1999. Late endosomal membranes rich in lysobisphosphatidic acid regulate cholesterol transport. Nat. Cell. Biol. 1: 113–118.
42. Cham, B. P., J. M. Gerrard, and D. F. Bainton. 1994. Granulophysin is located in the membrane of azurophilic granules in human neutrophils and mobilizes to the plasma membrane following cell stimulation. Am. J. Pathol. 144: 1369 –1380.
43. Kato, M., H. Kimura, Y. Motegi, A. Tachibana, H. Minakami, A. Morikawa, and H. Kita. 2002. Platelet-activating factor activates two distinct effector pathways in human eosinophils. J. Immunol. 169: 5252–5259.
44. Richardson, R. M., B. Haribabu, H. Ali, and R. Snyderman. 1996. Cross-desensitization among receptors for platelet activating factor and peptide chemoattractants: evidence for independent regulatory pathways. J. Biol. Chem. 271: 28717–28724.
45. Grady, E. F., S. K. Bohm, and N. W. Bunnett. 1997. Turning off the signal: mechanisms that attenuate signaling by G protein-coupled receptors. Am. J. Physiol. 273: G586–G601.
46. Solomon, W. R. 1975. Assessing fungus prevalence in domestic interiors. J. Allergy Clin. Immunol. 56: 235–242.
47. Mitakakis, T. Z., C. Barnes, and E. R. Tovey. 2001. Spore germination increases allergen release from Alternaria. J. Allergy Clin. Immunol. 107: 388 –390.
48. Halonen, M., D. A. Stern, A. L. Wright, L. M. Taussig, and F. D. Martinez. 1997. Alternaria as a major allergen for asthma in children raised in a desert environment. Am. J. Respir. Crit. Care Med. 155: 1356–1361.
49. Zureik M., C. Neukirch, B. Leynaert, R. Liard, J. Bousquet, F. Neukirch, and European Community Respiratory Health Survey. 2002. Sensitisation to airborne moulds and severity of asthma: cross sectional study from European Community
respiratory health survey. Br. Med. J. 325: 411–414.
50. Black, P. N., A. A. Udy, and S. M. Brodie. 2000. Sensitivity to fungal allergens is a risk factor for life-threatening asthma. Allergy 55: 501–504.
51. Kaufman, H. F., J. F. Tomee, M. A. van de Riet, A. J. Timmerman, and P. Borger. 2000. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J. Allergy Clin. Immunol. 105:
1185–1193.
52. Miike, S., A. S. McWillam, and H. Kita. 2001. Trypsin induces activation and inflammatory mediator release from human eosinophils through protease-activated receptor-2. J. Immunol. 167: 6615–6622.
53. Steinhoff, M., J. Buddenkotte, V. Shpacovitch, A. Rattenholl, C. Moormann, N. Vergnolle, T. A. Luger, and M. D. Hollenberg. 2005. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune
response. Endocr. Rev. 26: 1–43.
54. Al-Ani, B., M. Saifeddine, S. J. Wijesuriya, and M. D. Hollenberg. 2002. Modified proteinase-activated receptor-1 and -2 derived peptides inhibit proteinase-activated receptor-2 activation by trypsin. J. Pharmacol. Exp. Ther. 300: 702–708.
55. Williams, D. L. 1997. Overview of (133)--D-glucan immunobiology. Mediat. Inflamm. 6: 247–250.
56. D’Souza, M. P., J. S. Cairns, and S. F. Plaeger. 2000. Current evidence and future directions for targeting HIV entry: therapeutic and prophylactic strategies. J. Am. Med. Assoc. 284: 215–222.
57. Yoganathan, K., C. Rossant, S. Ng, Y. Huang, M. S. Butler, and A. D. Buss. 2003. 10-Methoxydihydrofuscin, fuscinarin, and fuscin, novel antagonists of the human CCR5 receptor from Oidiodendron griseum. J. Nat. Prod. 66: 1116 –117.
58. Romani, L. 2004. Immunity to fungal infections. Nat. Rev. Immunol. 4: 1–13.

 FIGURE 2. Alternaria-induced eosinophil degranulation appears regulated and exocytotic. Transmission photomicrographs show eosinophils after 3 h at 37°C of incubation with medium or Alternaria extract. A, Eosinophils incubated in medium only; note the wellmaintained cytoplasmic granules containing characteristic electron-dense core and matrix structures and intact plasma membranes. B, Eosinophils stimulated with 100 g/ml Alternaria culture extract show granule fusion and electron-lucent granules, but plasma membranes are intact. C and D, Higher magnification views of eosinophils incubated with Alternaria show the intact plasma membrane and emphasize the granule fusion and loss of electron-dense material from granules. Original magnification: A and B, 6000; C, 20,000; D, 60,000.
FIGURE 2. Alternaria-induced eosinophil degranulation appears regulated and exocytotic. Transmission photomicrographs show eosinophils after 3 h at 37°C of incubation with medium or Alternaria extract. A, Eosinophils incubated in medium only; note the wellmaintained cytoplasmic granules containing characteristic electron-dense core and matrix structures and intact plasma membranes. B, Eosinophils stimulated with 100 g/ml Alternaria culture extract show granule fusion and electron-lucent granules, but plasma membranes are intact. C and D, Higher magnification views of eosinophils incubated with Alternaria show the intact plasma membrane and emphasize the granule fusion and loss of electron-dense material from granules. Original magnification: A and B, 6000; C, 20,000; D, 60,000.